Table of Contents
Glycation of proteins is a natural process that enhances bio lubrication at soft-to-hard tissue interfaces which is crucial for biological surfaces like the oral cavity, eyes, and joints. Glycoproteins are the primary lubricants on these surfaces that protects the surfaces by reducing friction. For example, in the case of eyes, protein glycation helps form a lubricious tear film which safe guards the eye from various external dust and dirt etc. Studies show that glycation improves the viscosity of synthetic lubricants and further in tablets it enhances the lubrication of oral dosage forms by reducing equipment friction. In foods the controlled glycation reactions enhances the stability of protein-stabilized emulsions which protects them from coalescence and flocculation under various conditions. This process affects food rheology which includes viscosity and flow behavior and improves tribological properties like surface interactions. Further, understanding glycation’s tribological effects could lead to food innovations with reduced calories or higher protein content without affecting the mouthfeel and by tailored oral processing techniques. Thus, glycation is one of the important aspects that attracts the bio-tribologists to explore more.
Figure-1 Schematic illustration of overview of application of glycation
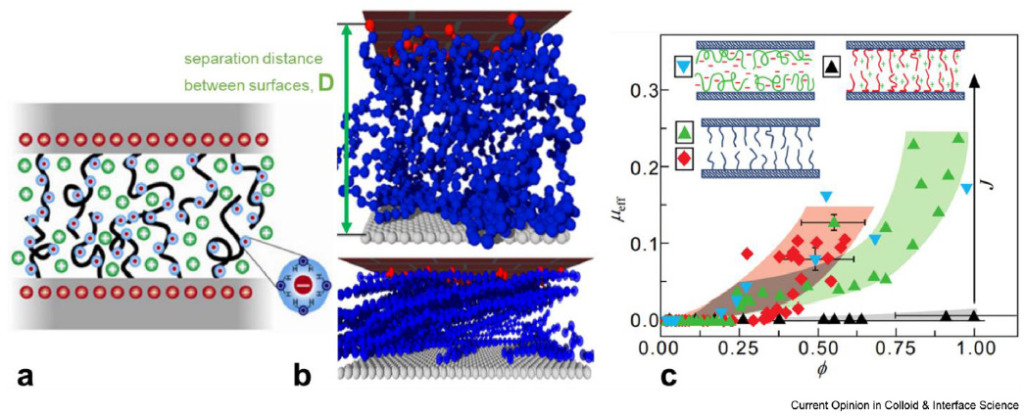
Biological fluids like synovial fluid, tears, and saliva serve as natural lubricants helps in reducing the friction and wear between tissues. These fluids can achieve Superlubricity, with extremely low friction coefficients (<0.001) an act as bio or natural lubricants. This effect is largely due to glycosylated proteins, like mucins and lubricins, whose glycan-rich, brush-like structures provide efficient lubrication even under high physiological pressures (10 MPa or more). This lubrication is studied intensively by Klein et. al.

Figure-2 Hydration lubrication mechanism in brush-like polymers evidenced via simulation and experimental approaches
Under low to moderate compression the glycan brushes on biological surfaces experience minimal entanglement due to “steric-entropic” repulsion which allows them to slide past each other and bear load effectively. The molecular dynamics simulations shows that dextran chains generate repulsive forces like hydrodynamic separation which enables smooth sliding without a boundary regime. Under these conditions, glycans help to maintain a non-interpenetrating fluid-like layer by allowing water molecules to move freely with minimal friction. Further, the trapped counter-ions in hydrated shells also increase osmotic pressure, enhancing this lubrication. Similarly at higher compressions, when hydrated glycans overlaps the entropic effects become less relevant, and water shells around glycans shift to a hydration lubrication mechanism.
Figure-3 Schematic illustrations of key glycosylated structures in biology acting as high-performance nature-engineered lubricants